Difference between revisions of "The Science Of Hydration"
User:Fellrnr (User talk:Fellrnr | contribs) m (→Sodium loss through sweat) |
User:Fellrnr (User talk:Fellrnr | contribs) |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | = Introduction = | + | The science of hydration for exercise is complex and controversial. While dehydration can cause problems, the real risk is [[Hyponatremia]] where the level of sodium in the blood is too low. The science shows that sodium losses increase exponentially with sweat rate, so exercise in the heat can result in extreme levels of sodium loss. For a simpler look at hydration, see [[Practical Hydration]]. |
| − | The advice given to runners on hydration has changed over time and looks set to continue to change. There are competing forces at work - sports drink manufacturers, event organizers (often sponsored by the manufacturers) and scientists (some also sponsored by the manufacturers). One thing is clear about hydration - it is important. Incorrect hydration can lead to impaired performance, and in extreme cases, death. A condition related to dehydration is [[Hyponatremia]], which is where the sodium (salt) level in the blood becomes too dilute. This is a dangerous condition that has killed a number of runners. | + | =Introduction= |
| − | + | The advice given to runners on hydration has changed over time and looks set to continue to change. There are competing forces at work - sports drink manufacturers, event organizers (often sponsored by the manufacturers) and scientists (some also sponsored by the manufacturers). One thing is clear about hydration - it is important. Incorrect hydration can lead to impaired performance, and in extreme cases, death. A condition related to dehydration is [[Hyponatremia]], which is where the sodium (salt) level in the blood becomes too dilute. This is a dangerous condition that has killed a number of runners. This entry is a follow on to [[Practical Hydration]] which should be read first. | |
| − | This entry is a follow on to [[Practical Hydration]] which should be read first. | + | =Effects of dehydration= |
| − | + | While it's commonly believed that even small levels of dehydration impacts performance, the research indicates that real world performance is not impacted by dehydration up to 4% of body weight<ref name="Goulet2012"/><ref name="Goulet2011"/>. Greater levels of dehydration will impact performance<ref name="González-Alonso-1995"/>. While authorities have recommended aggressive drinking<ref name="Convertino-1996"/>, these guidelines are now recognized as erroneous<ref name="WallWatson2013"/>, though they are still promoted by the beverage industry<ref name="beverageWWW"/>. | |
| − | = Effects of dehydration = | + | =Glycogen Depletion, Dehydration and Body Weight = |
| − | + | A common method of calculating dehydration is simply from body weight. While it's true that an athlete's weight loss during exercise will be predominantly from water, this is not the same as dehydration, nor does it necessarily imply this weight needs to be restored quickly though drinking. This is because a carbohydrate ([[Glycogen]]) is stored with water, in the ratio of about 1g [[Glycogen]] to 3-4g water<ref name="OlssonSaltin1970"/><ref name="Nilsson-1973"/>. This means that 2000 calories of [[Glycogen]] depletion that are likely to occur in marathon distance runs would result in about 4lb/2Kg [[Weight Loss]] with no reduction in hydration (2000Kcal/4=500g [[Glycogen]] + 1500g to 2000g water = 2000g to 2500g). To restore the weight lost in endurance exercise the Glycogen reserves must also be restored, something that may take days. Even moving from a high carbohydrate to high fat diet can see 6lb [[Weight Loss]] from [[Glycogen]] depletion. | |
| − | + | =Sodium Intake and Rehydration= | |
| − | = Sodium loss through sweat = | + | Several studies have shown that drinks containing sodium provide better rehydration by reducing urine output. |
| − | The amount of salt that is lost through sweating varies a lot. It varies from individual to individual, and for an individual it will vary depending on fitness and heat acclimation | + | * Consuming drinks with 61 mmol/l (~1/2 teaspoon salt per quart/liter) reduced urine output and improved hydration when compared with 23 mmol/l (~1/4 teaspoon salt per quart/liter)<ref name="Shirreffs-1996"/> |
| − | + | * When comparing drinks with 2, 26, 52 and 100 mmol/l, urine output was inversely proportional to sodium concentration, and the 2 & 26 mmol/l cases resulting in dehydrated subjects after 5.5 hours, where the others were rehydrated<ref name="Maughan-1995"/>. (These cases are roughly equal to water, 1/4, 1/2, 3/4 teaspoon of salt per quart/liter.) | |
| + | * Drinks containing 0.45g salt/100ml reduced urine output and improved hydration<ref name="Nose-1988"/>. (0.45g/100ml is about 1/10 teaspoon of salt per quart/liter.) | ||
| + | =Sodium loss through sweat= | ||
| + | The amount of salt that is lost through sweating varies a lot. It varies from individual to individual, and for an individual it will vary depending on fitness and heat acclimation. This means that you may have to experiment with your salt intake, both during and after exercise. | ||
| + | ==Anatomy of Sweating== | ||
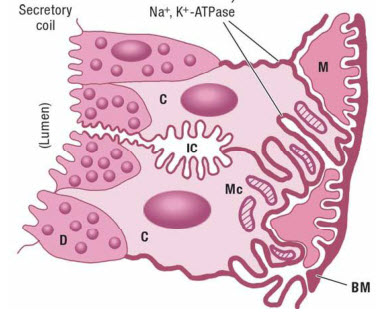
| + | [[File:Sweat Gland.jpg|right|thumb|500px|A drawing of the sweat gland. (C=clear cells, D=dark cells, IC=Intracellular canaliculi, M=Myoepitherial cell, Mc=Mitochondria. )]] | ||
| + | Humans have 2-4 million sweat glands over nearly their whole body surface, and though each is tiny, together they weigh as much as a kidney (~100g)<ref name="Fitzpatrick"/>. Sweat glands are most numerous on the soles of the feet. Sweat is produced in two steps<ref name="Fitzpatrick"/>: | ||
| + | # A coil generates an ultra-filtrated fluid. This fluid has the same sodium concentration (isotonic) as the blood. | ||
| + | # The fluid travels from the coil up the sweat duct which reabsorbs sodium and chloride (but not [[Potassium|potassium]]). This reabsorption is via active transportation (i.e. it requires energy from ATP). The [[Glycogen]] stored in the sweat glands will only support the reabsorption for less than 10 min., so the energy is predominantly supplied by the blood. Glucose is the preferred energy source, though [[Lactate]] and pyruvate can also be used. Fatty acids, ketones, and amino acids are very poorly used. The reabsorption process also acidifies the final sweat. | ||
| + | The rate of sweat production depends on the local skin temperature and core body temperature. A rise in the localized skin temperature will produce an increased sweat rate within 60 seconds<ref name="Fitzpatrick"/>. | ||
==Sodium Loss Table== | ==Sodium Loss Table== | ||
The table below is based on the research showing that sweat sodium concentration increases with sweat rate. The table below is for a runner who is 174cm/70inches high and weighs 60Kg/132lbs, but you can create a customized chart at [[Sodium Loss]]. To check your sweat rate, simply weigh yourself before and after a run. Dropping 1 Kg or 2.2 pounds equates to 1 liter of sweating. (Obviously you need to adjust for any fluid intake and avoid urination.) | The table below is based on the research showing that sweat sodium concentration increases with sweat rate. The table below is for a runner who is 174cm/70inches high and weighs 60Kg/132lbs, but you can create a customized chart at [[Sodium Loss]]. To check your sweat rate, simply weigh yourself before and after a run. Dropping 1 Kg or 2.2 pounds equates to 1 liter of sweating. (Obviously you need to adjust for any fluid intake and avoid urination.) | ||
| − | {| class="wikitable" | + | {| class="wikitable" style="margin-left: auto; margin-right: auto; border: none;" |
| + | ! | ||
| + | ! | ||
| + | ! colspan="3"|'''Heat Acclimated''' | ||
| + | ! colspan="3"|'''Heat Non-Acclimated''' | ||
|- | |- | ||
| − | | | + | | '''Sweat Rate''' |
| − | + | | '''Sweat Rate''' | |
| − | + | | '''Sweat Concentration''' | |
| − | | | + | | [[Sodium Loss]] |
| − | + | | [[Sodium Loss]] | |
| − | + | | '''Sweat Concentration''' | |
| − | | | + | | [[Sodium Loss]] |
| − | + | | [[Sodium Loss]] | |
| − | |||
| − | | | ||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | '''(l/hr)''' |
| − | + | | '''(mg/cm2/min)''' | |
| − | + | | '''(mmol/l)''' | |
| − | | | + | | '''(mg/hr)''' |
| − | + | | '''(tsp/hr)''' | |
| − | + | | '''(mmol/l)''' | |
| − | | | + | | '''(mg/hr)''' |
| − | + | | '''(tsp/hr)''' | |
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | '''0.5''' |
| − | + | | '''0.49''' | |
| − | + | | 22 | |
| − | | | + | | 249 |
| − | + | | 0.1 | |
| − | + | | 31 | |
| − | | | + | | 355 |
| − | + | | 0.2 | |
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | '''1''' |
| − | + | | '''0.98''' | |
| − | + | | 32 | |
| − | | | + | | 732 |
| − | + | | 0.3 | |
| − | + | | 46 | |
| − | | | + | | 1044 |
| − | + | | 0.4 | |
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | '''1.5''' |
| − | + | | '''1.47''' | |
| − | + | | 43 | |
| − | | | + | | 1450 |
| − | + | | 0.6 | |
| − | + | | 61 | |
| − | | | + | | 2066 |
| − | + | | 0.9 | |
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | '''2''' |
| − | + | | '''1.96''' | |
| − | + | | 53 | |
| − | | | + | | 2402 |
| − | + | | 1 | |
| − | + | | 75 | |
| − | | | + | | 3423 |
| − | + | | 1.5 | |
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | | '''2.5''' |
| − | + | | '''2.45''' | |
| − | + | | 63 | |
| − | + | | 3589 | |
| − | + | | 1.5 | |
| − | + | | 90 | |
| − | + | | 5113 | |
| − | + | | 2.2 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
|} | |} | ||
| − | + | This table is based on the research quoted below showing a linear relationship between sweat rate and sweat sodium concentration. | |
| − | This table is based on the research quoted below showing a linear relationship between sweat rate | ||
| − | |||
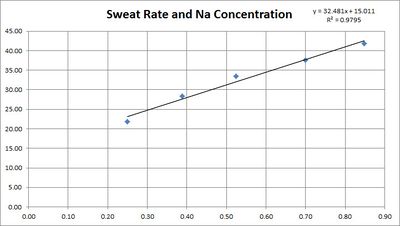
==Sodium Loss and Sweat Rate== | ==Sodium Loss and Sweat Rate== | ||
The concentration of sodium in sweat depends on the sweat rate. This is believed to be because the sweat is released with a high sodium concentration, then the sodium is reabsorbed before it reaches the surface. The faster the sweating, the less chance for reabsorption. | The concentration of sodium in sweat depends on the sweat rate. This is believed to be because the sweat is released with a high sodium concentration, then the sodium is reabsorbed before it reaches the surface. The faster the sweating, the less chance for reabsorption. | ||
| − | [[File:Sweat Rate Sodium Concentration.jpg|none|thumb| | + | [[File:Sweat Rate Sodium Concentration - adjusted.jpg|none|thumb|400px|Sweat rate and sodium concentration<ref name="Buono-2008"/>, adjusted using the formula for regional patch collection<ref name="Baker-2009"/>.]] |
| + | ===Converting per-area sweat rates to whole body sweat rates=== | ||
We can convert from per-area sweat rates to whole body sweat rates by using a [http://www.halls.md/body-surface-area/bsa.htm Body Surface Area Calculator]. For example, a 135 pound, 70 inch high person has a skin surface area of 1.74 m<sup>2</sup>, which is 17,400 cm<sup>2</sup>. Therefore 1 mg/cm<sup>2</sup>/min is 17,400 mg/min, or 17.4 g/min or 1,044 g/hour, or 1 liter/hour. | We can convert from per-area sweat rates to whole body sweat rates by using a [http://www.halls.md/body-surface-area/bsa.htm Body Surface Area Calculator]. For example, a 135 pound, 70 inch high person has a skin surface area of 1.74 m<sup>2</sup>, which is 17,400 cm<sup>2</sup>. Therefore 1 mg/cm<sup>2</sup>/min is 17,400 mg/min, or 17.4 g/min or 1,044 g/hour, or 1 liter/hour. | ||
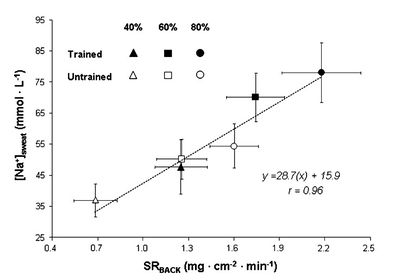
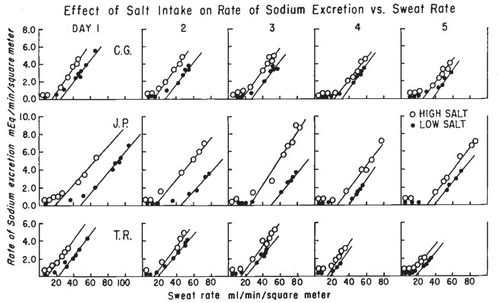
==Sodium Loss and Fitness== | ==Sodium Loss and Fitness== | ||
| − | While some sources suggest that increased fitness reduces the sodium concentration in sweat research<ref name=" | + | While some sources suggest that increased fitness reduces the sodium concentration in sweat research<ref name="Hamouti-2011"/> shows this is not the case. For both trained and untrained individuals sodium concentration depends mainly on sweat rate. In fact, for a given relative intensity (% of [[VO2max|V̇O<sub>2</sub>max]]) trained individuals will be performing a greater absolute work rate and therefore have a greater sweat rate and sodium concentration. |
| − | [[File:Sodium in sweat trained and untrained.jpg|none|thumb| | + | [[File:Sodium in sweat trained and untrained.jpg|none|thumb|400px|Sweat sodium concentration against sweating rate, showing for three different work intensities and for trained and untrained individuals. Note that this data is not adjusted for regional patch collection, so the rates are too high and should be scaled by 0.67.]] |
| − | |||
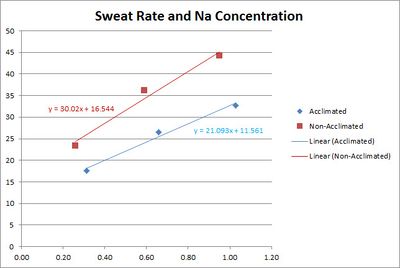
==Sodium Loss and Heat Acclimation== | ==Sodium Loss and Heat Acclimation== | ||
| − | A study<ref name=" | + | A study<ref name="Buono-2007"/> shows that the sodium concentration of sweat is reduced by [[Heat Acclimation Training]]. The study used three bouts of 30 min. of exercise in environmental chamber with 10 min. of rest between each bout. |
| − | [[File:Sweat Rate Sodium Concentration for heat adaptation.jpg|none|thumb| | + | [[File:Sweat Rate Sodium Concentration for heat adaptation - adjusted.jpg|none|thumb|400px|Sweat sodium concentration against sweating rate, before and after 10 days of heat acclimation training, adjusted using the formula for regional patch collection<ref name="Baker-2009"/>.]] |
| − | |||
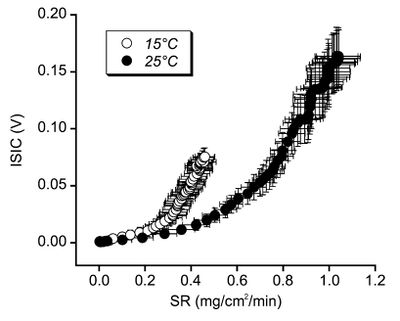
==Sodium Loss and Skin Temperature== | ==Sodium Loss and Skin Temperature== | ||
| − | A study<ref name=" | + | A study<ref name="Shamsuddin-2005"/> of sweating great sodium concentration for different temperatures has shown that sodium reabsorption is greater at high temperatures. Unfortunately the units used in this study are not comparable with other studies. The mechanism behind this is unclear, but the implication is that the sodium concentration of sweat in cooler weather may be higher than expected from the above studies. |
| − | [[File:Sweat Rate Sodium Concentration for skin temperatures.jpg|none|thumb| | + | [[File:Sweat Rate Sodium Concentration for skin temperatures.jpg|none|thumb|400px|Sweat sodium concentration against sweating great, shown for two different skin temperatures.]] |
| − | |||
=Sodium Retention= | =Sodium Retention= | ||
| − | The human body | + | The human body has mechanisms to try to maintaining its sodium balance. Greater sodium intake results in the excess being excreted in the sweat and urine. Conversly, restricted sodium intake will result in a reduction of the sodium concentration of the sweat<ref name="CONN-1946"/>. This reduction in the sodium concentration occurs at all sweating rates, but the relationship between sodium concentration and sweat rate remains a straight line<ref name="Sigal-1968"/>. So at any given sweat rate, a restricted sodium intake will result in less sodium in the sweat. However, even on a restricted sodium intake, the more you sweat, the greater the sodium concentration. Comparing the maximum sodium concentration of sweat between a salt intake of 500mg/day and 20,000mg/day, the low salt intake reduced the sodium concentration by 30-48%<ref name="Sigal-1968"/>. |
| − | + | [[File:SodiumIntakeSweatConcentration.jpg|none|thumb|500px|The effect of high (20,000mg/day NaCL) and low (500mg/day NaCL) on the sodium concentration of sweat at various sweat rates<ref name="Sigal-1968"/>. Three subjects were put on the diet for a week, then tested for five days while remaining on that diet. The two tests were separated by a month.]] | |
=Sodium Intake= | =Sodium Intake= | ||
Below are some sample sources of Sodium, with the concentrations defined. | Below are some sample sources of Sodium, with the concentrations defined. | ||
| − | {| | + | {| class="wikitable" style="margin-left: auto; margin-right: auto; border: none;" |
| − | + | ! Source | |
| − | + | ! Sodium - mmol per liter | |
| − | + | ! Sodium - grams per liter | |
| − | + | ! Sodium - grams per pint | |
| − | + | ! Salt - grams per pint | |
|- | |- | ||
| − | |Gatorade | + | | Gatorade |
| + | | 18 | ||
| + | | 0.4 | ||
| + | | 0.2 | ||
| + | | 0.5 | ||
|- | |- | ||
| − | |Water + 1/4 Teaspoon salt per quart | + | | Water + 1/4 Teaspoon salt per quart |
| + | | 27 | ||
| + | | 0.6 | ||
| + | | 0.3 | ||
| + | | 0.75 | ||
|- | |- | ||
| − | |Gatorade+ 1/4 Teaspoon salt per quart | + | | Gatorade+ 1/4 Teaspoon salt per quart |
| + | | 45 | ||
| + | | 1.0 | ||
| + | | 0.5 | ||
| + | | 1.2 | ||
|- | |- | ||
| − | |S-Caps + 8oz water* | + | | S-Caps + 8oz water* |
| + | | 65 | ||
| + | | 1.4 | ||
| + | | 0.7 | ||
| + | | 1.7 | ||
|- | |- | ||
| − | |Salt Stick + 8oz Water | + | | Salt Stick + 8oz Water |
| + | | 38 | ||
| + | | 0.84 | ||
| + | | 0.4 | ||
| + | | 0.98 | ||
|- | |- | ||
| − | |Salt Stick + 16oz Water | + | | Salt Stick + 16oz Water |
| + | | 19 | ||
| + | | 0.42 | ||
| + | | 0.2 | ||
| + | | 0.49 | ||
|} | |} | ||
| − | + | Note: S-Caps does not specify the amount of fluid to take with each capsule, but does mention 'at least one cup', so this ratio is used. The per-pint and per-liter equivalents assume a constant ratio of one capsule per 8oz of water. See also [[Comparison of Gels]]. | |
| − | Note: S-Caps does not specify the amount of fluid to take with each capsule, but does mention 'at least one cup', so this ratio is used. The per-pint and per-liter equivalents | ||
| − | |||
| − | See also [[Comparison of Gels]]. | ||
| − | |||
==Example Sodium Losses== | ==Example Sodium Losses== | ||
Here are some hypothetical examples | Here are some hypothetical examples | ||
| − | |||
* Adam, a heat acclimatized runner, weighs himself before and after his four hour run and the difference is 8 pounds, which is roughly equivalent to 8 pints/4 liters of sweat. Based on 1 liter/hour of sweating we estimate Adam lost 4 grams of sodium, which is about 2 teaspoons. | * Adam, a heat acclimatized runner, weighs himself before and after his four hour run and the difference is 8 pounds, which is roughly equivalent to 8 pints/4 liters of sweat. Based on 1 liter/hour of sweating we estimate Adam lost 4 grams of sodium, which is about 2 teaspoons. | ||
* Bob is not heat acclimatized runner, and losses 9 pounds in three hours (9 pints/4.5 liters). From the sweat rate we estimate that Bob has lost 7.5 grams of sodium, which is about 3.3 teaspoons. | * Bob is not heat acclimatized runner, and losses 9 pounds in three hours (9 pints/4.5 liters). From the sweat rate we estimate that Bob has lost 7.5 grams of sodium, which is about 3.3 teaspoons. | ||
| − | |||
=Sweat Rates While Running= | =Sweat Rates While Running= | ||
Sweat rates in male runners have been measured in the range from 0.75-2.23 in winter to 0.99-2.55 in the summer (Liters per hour)<ref name="acsm"/>. At the low end, we can imagine a fit runner finishing a 3-hour marathon in winter and sweating only 2.25 Liters. Assuming they are also heat acclimated, they would only lose 2 grams of sodium, which is 5 grams of salt, less than a teaspoon. On the other end of the scale, a fit, but unacclimatized runner completing a 5 hour marathon in summer would sweat out nearly 13 Liters, 18 grams of sodium, which is 45 grams of salt or more than 7 teaspoons. | Sweat rates in male runners have been measured in the range from 0.75-2.23 in winter to 0.99-2.55 in the summer (Liters per hour)<ref name="acsm"/>. At the low end, we can imagine a fit runner finishing a 3-hour marathon in winter and sweating only 2.25 Liters. Assuming they are also heat acclimated, they would only lose 2 grams of sodium, which is 5 grams of salt, less than a teaspoon. On the other end of the scale, a fit, but unacclimatized runner completing a 5 hour marathon in summer would sweat out nearly 13 Liters, 18 grams of sodium, which is 45 grams of salt or more than 7 teaspoons. | ||
| − | |||
There is a table showing a range of values at [[Sodium Loss]]. | There is a table showing a range of values at [[Sodium Loss]]. | ||
| − | + | =Hyponatremia= | |
| − | = Hyponatremia = | + | [[Hyponatremia]] is where the sodium (salt) levels in the blood become too dilute. The symptoms of mild hyponatremia tend to be a gain in weight and a general swelling and 'puffiness', most noticeable in the hands. However, mild Hyponatremia may have no clinical symptoms, or just weakness, dizziness, headache, nausea/vomiting, but more severe Hyponatremia is likely to have symptoms of cerebral edema, including altered mental status, seizures, pulmonary edema, coma, and death<ref name="RosnerKirven2006"/>. |
| − | [[Hyponatremia]] is where the sodium (salt) levels | + | =HypERnatremia - the opposite of HypOnatremia= |
| − | = HypERnatremia - the opposite of HypOnatremia = | + | Generally, Hypernatremia (too much sodium in the blood) seems to be a result of dehydration rather than excessive salt intake<ref name="Kratz-2005"/>. It should be noted that taking [[Electrolyte Capsules]] bypasses the body's taste. This sense of taste seems to reflect our body's internal sensors; our desire for salty foods reflects our salt requirements. |
| − | Generally, Hypernatremia (too much sodium in the blood) seems to be a result of dehydration rather than excessive salt intake <ref name=" | + | =Salt and High Blood Pressure= |
| − | = Salt and High Blood Pressure = | + | There is evidence that increased salt intake can increase blood pressure<ref name="ref4"/>, and the common recommendation is to restrict your salt intake if you have high blood pressure. However, a recent study<ref name="Stolarz-Skrzypek-2011"/> has shown that reducing your salt intake may increase your risk of a heart attack rather than lower it. For more on the health risks of low salt diets see http://www.drmirkin.com/public/ezine050811.html |
| − | There is evidence that increased salt intake can increase blood pressure <ref name="ref4"/>, and the common recommendation is to restrict your salt intake if you have high blood pressure. However, a recent study<ref name=" | ||
| − | |||
As an aside, if you have low blood pressure, which I do, increasing your salt intake can really help. | As an aside, if you have low blood pressure, which I do, increasing your salt intake can really help. | ||
| − | = Caffeine | + | =Caffeine= |
| − | The scientific evidence shows that | + | The scientific evidence shows that [[Caffeine]] is generally not a diuretic<ref name="Graham-1998"/><ref name="Falk-1990"/>. Previous studies have shown that if you don't normally take [[Caffeine]] and then get a large dose, there is some diuretic effect. However normal intakes of [[Caffeine]] by non-users and use by regular users is not a diuretic<ref name="Maughan-2003"/>. (If you urinate more because you drink a 20oz Latte, it is because of the 20oz of fluid, not the [[Caffeine]].) |
| − | Alcohol is | + | =Alcohol= |
| − | = Cramps = | + | Alcohol is a diuretic due to the suppression of vasopressin secretion<ref name="ROBERTS-1963"/><ref name="RubiniKleeman1955"/>, and the volume of urine produced is proportional to the alcohol consumed<ref name="Eggleton-1942"/>. However, the diuretic effect of alcohol may be reduced when consumed when suffering from post-exercise dehydration. A study looked at consuming water or an alcoholic beverage of 1%, 2%, and 4% after 1 hour of moderate exercise<ref name="Shirreffs-1997"/>. The study found that urine production tended to increase with alcohol content, but the difference was not statistically significant. The alcohol tended to reduce the rate of recovery for both blood and plasma volume, though tendency was only statistically significant at 4%. In summary, alcoholic beverages of up to 2% appear to have little impact on rehydration rate compared with the equivalent volume of water, but 4% beverages tend to delay recovery. |
| + | =Cramps = | ||
The evidence for hydration and electrolyte status causing [[Cramps]] is somewhat ambiguous, but supplementing your electrolyte intake may help. | The evidence for hydration and electrolyte status causing [[Cramps]] is somewhat ambiguous, but supplementing your electrolyte intake may help. | ||
| − | = Blisters and black toe nails = | + | =Blisters and black toe nails= |
| − | Dehydration reduces body weight, which can reduce the size of your feet. This in turn changes the fit of your | + | Dehydration reduces body weight, which can reduce the size of your feet. This in turn changes the fit of your [[Shoes]], causing blisters. [[Hyponatremia]] can cause swelling, which increases the size of your feet and can cause blisters. Both conditions can also increase the chance of black toe nails. |
| − | =Sodium and Water in the Body | + | =Sodium and Water in the Body= |
| − | Approximately 60% of the human body weight is water, though this varies primarily with body fat as adipose (fat) tissue contains a lower percentage of water. Total Body Water (TBW) can be divided up into | + | Approximately 60% of the human body weight is water, though this varies primarily with body fat as adipose (fat) tissue contains a lower percentage of water. Total Body Water (TBW) can be divided up into<ref name="CLINC"/>: |
* Intracellular fluid (ICF) which is 40% of body weight | * Intracellular fluid (ICF) which is 40% of body weight | ||
* Extracellular fluid (ECF) which is the other 20% of body weight | * Extracellular fluid (ECF) which is the other 20% of body weight | ||
| − | * | + | * Plasma is 25% of ECF/5% body weight |
| − | * | + | * Interstitial fluid which is 75% of ECF/15% of body weight, typically 11 Liters/22 pints. |
The volume of extracellular fluid is typically 15 liters in a 70 kg human, and the 50 grams of sodium it contains is about 90% of the body's total sodium content. | The volume of extracellular fluid is typically 15 liters in a 70 kg human, and the 50 grams of sodium it contains is about 90% of the body's total sodium content. | ||
| − | + | =Symptoms of Dehydration= | |
| − | =Symptoms of Dehydration<ref name="CLINC"/> | + | These symptoms are for the general public<ref name="CLINC"/>, and there is evidence<ref name="SYMPT"/> that they may not apply to athletes suffering from dehydration. |
| − | + | {| class="wikitable" style="margin-left: auto; margin-right: auto; border: none;" | |
| − | {| | + | ! symptom |
| − | + | ! mild dehydration (3-5% body weight) | |
| − | + | ! Moderate dehydration (6-9% body weight) | |
| − | + | ! Severe dehydration (>10% body weight) | |
| − | |||
|- | |- | ||
| − | | Level of consciousness | + | | Level of consciousness |
| + | | Alert | ||
| + | | Lethargic | ||
| + | | Obtunded | ||
|- | |- | ||
| − | | Capillary Refill | + | | Capillary Refill |
| + | | 2 seconds | ||
| + | | 2-4 seconds | ||
| + | | >4 seconds | ||
|- | |- | ||
| − | | Blood Pressure | + | | Blood Pressure |
| + | | Normal | ||
| + | | Normal supine, lower standing | ||
| + | | lower | ||
|- | |- | ||
| − | | Skin Turgor | + | | Skin Turgor |
| + | | Normal | ||
| + | | Slow | ||
| + | | Tenting | ||
|- | |- | ||
| − | | Eyes | + | | Eyes |
| + | | Normal | ||
| + | | Sunken | ||
| + | | Very Sunken | ||
|} | |} | ||
| − | = References = | + | =References = |
<references> | <references> | ||
| + | <ref name="RosnerKirven2006">M. H. Rosner, J. Kirven, Exercise-Associated Hyponatremia, Clinical Journal of the American Society of Nephrology, volume 2, issue 1, 2006, pages 151–161, ISSN [http://www.worldcat.org/issn/1555-9041 1555-9041], doi [http://dx.doi.org/10.2215/CJN.02730806 10.2215/CJN.02730806]</ref> | ||
<ref name="CLINC">Clinical Studies in Fluid and Electrolyte Balance</ref> | <ref name="CLINC">Clinical Studies in Fluid and Electrolyte Balance</ref> | ||
| − | + | <ref name="SYMPT">J. McGarvey, J. Thompson, C. Hanna, T. D. Noakes, J. Stewart, D. Speedy, Sensitivity and specificity of clinical signs for assessment of dehydration in endurance athletes, British Journal of Sports Medicine, volume 44, issue 10, 2008, pages 716–719, ISSN [http://www.worldcat.org/issn/0306-3674 0306-3674], doi [http://dx.doi.org/10.1136/bjsm.2008.053249 10.1136/bjsm.2008.053249]</ref> | |
| − | + | <ref name="Nilsson-1973">LH. Nilsson, Liver glycogen content in man in the postabsorptive state., Scand J Clin Lab Invest, volume 32, issue 4, pages 317-23, Dec 1973, PMID [http://www.ncbi.nlm.nih.gov/pubmed/4771101 4771101]</ref> | |
| − | <ref name="SYMPT">Sensitivity and specificity of clinical signs for assessment of dehydration in endurance athletes | + | <ref name="González-Alonso-1995">J. González-Alonso, R. Mora-Rodríguez, PR. Below, EF. Coyle, Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise., J Appl Physiol (1985), volume 79, issue 5, pages 1487-96, Nov 1995, PMID [http://www.ncbi.nlm.nih.gov/pubmed/8594004 8594004]</ref> |
| − | + | <ref name="Graham-1998">TE. Graham, E. Hibbert, P. Sathasivam, Metabolic and exercise endurance effects of coffee and caffeine ingestion., J Appl Physiol (1985), volume 85, issue 3, pages 883-9, Sep 1998, PMID [http://www.ncbi.nlm.nih.gov/pubmed/9729561 9729561]</ref> | |
| − | + | <ref name="Falk-1990">B. Falk, R. Burstein, J. Rosenblum, Y. Shapiro, E. Zylber-Katz, N. Bashan, Effects of caffeine ingestion on body fluid balance and thermoregulation during exercise., Can J Physiol Pharmacol, volume 68, issue 7, pages 889-92, Jul 1990, PMID [http://www.ncbi.nlm.nih.gov/pubmed/2383801 2383801]</ref> | |
| − | + | <ref name="Maughan-2003">RJ. Maughan, J. Griffin, Caffeine ingestion and fluid balance: a review., J Hum Nutr Diet, volume 16, issue 6, pages 411-20, Dec 2003, PMID [http://www.ncbi.nlm.nih.gov/pubmed/19774754 19774754]</ref> | |
| − | + | <ref name="Kratz-2005">A. Kratz, AJ. Siegel, JG. Verbalis, MM. Adner, T. Shirey, E. Lee-Lewandrowski, KB. Lewandrowski, Sodium status of collapsed marathon runners., Arch Pathol Lab Med, volume 129, issue 2, pages 227-30, Feb 2005, doi [http://dx.doi.org/10.1043/1543-2165(2005)129<227:SSOCMR>2.0.CO;2 10.1043/1543-2165(2005)129<227:SSOCMR>2.0.CO;2], PMID [http://www.ncbi.nlm.nih.gov/pubmed/15679427 15679427]</ref> | |
| − | [http://www. | ||
| − | |||
| − | |||
| − | |||
| − | [http:// | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | </ref> | ||
| − | |||
| − | <ref name=" | ||
| − | [http://www. | ||
| − | </ref> | ||
| − | |||
| − | <ref name=" | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | </ref> | ||
| − | |||
| − | |||
| − | |||
| − | <ref name=" | ||
| − | |||
| − | </ref> | ||
| − | |||
| − | <ref name=" | ||
| − | [http://www.ncbi.nlm.nih.gov/pubmed/2383801 | ||
| − | </ref> | ||
| − | |||
| − | <ref name=" | ||
| − | [http:// | ||
| − | </ref> | ||
| − | |||
| − | <ref name=" | ||
| − | [http:// | ||
| − | </ref> | ||
| − | |||
<ref name="acsm">http://journals.lww.com/acsm-msse/_layouts/oaks.journals/ImageView.aspx?k=acsm-msse:2007:02000:00022&i=TT2 | <ref name="acsm">http://journals.lww.com/acsm-msse/_layouts/oaks.journals/ImageView.aspx?k=acsm-msse:2007:02000:00022&i=TT2 | ||
</ref> | </ref> | ||
| − | |||
<ref name="ref4">Micronutrient Information Center - Sodium | <ref name="ref4">Micronutrient Information Center - Sodium | ||
http://lpi.oregonstate.edu/infocenter/minerals/sodium/ | http://lpi.oregonstate.edu/infocenter/minerals/sodium/ | ||
</ref> | </ref> | ||
| − | + | <ref name="Stolarz-Skrzypek-2011">K. Stolarz-Skrzypek, T. Kuznetsova, L. Thijs, V. Tikhonoff, J. Seidlerová, T. Richart, Y. Jin, A. Olszanecka, S. Malyutina, Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion., JAMA, volume 305, issue 17, pages 1777-85, May 2011, doi [http://dx.doi.org/10.1001/jama.2011.574 10.1001/jama.2011.574], PMID [http://www.ncbi.nlm.nih.gov/pubmed/21540421 21540421]</ref> | |
| − | <ref name=" | + | <ref name="Buono-2008">MJ. Buono, R. Claros, T. Deboer, J. Wong, Na+ secretion rate increases proportionally more than the Na+ reabsorption rate with increases in sweat rate., J Appl Physiol (1985), volume 105, issue 4, pages 1044-8, Oct 2008, doi [http://dx.doi.org/10.1152/japplphysiol.90503.2008 10.1152/japplphysiol.90503.2008], PMID [http://www.ncbi.nlm.nih.gov/pubmed/18653750 18653750]</ref> |
| − | http://jama. | + | <ref name="Hamouti-2011">N. Hamouti, J. Del Coso, JF. Ortega, R. Mora-Rodriguez, Sweat sodium concentration during exercise in the heat in aerobically trained and untrained humans., Eur J Appl Physiol, volume 111, issue 11, pages 2873-81, Nov 2011, doi [http://dx.doi.org/10.1007/s00421-011-1911-6 10.1007/s00421-011-1911-6], PMID [http://www.ncbi.nlm.nih.gov/pubmed/21431880 21431880]</ref> |
| − | </ref> | + | <ref name="CONN-1946">JW. CONN, MW. JOHNSTON, LH. LOUIS, Relationship between salt intake and sweat salt concentration under conditions of hard work in humid heat., Fed Proc, volume 5, issue 1 Pt 2, pages 230, 1946, PMID [http://www.ncbi.nlm.nih.gov/pubmed/20984571 20984571]</ref> |
| − | + | <ref name="Shamsuddin-2005">AK. Shamsuddin, T. Kuwahara, A. Oue, C. Nomura, S. Koga, Y. Inoue, N. Kondo, Effect of skin temperature on the ion reabsorption capacity of sweat glands during exercise in humans., Eur J Appl Physiol, volume 94, issue 4, pages 442-7, Jul 2005, doi [http://dx.doi.org/10.1007/s00421-005-1354-z 10.1007/s00421-005-1354-z], PMID [http://www.ncbi.nlm.nih.gov/pubmed/15843956 15843956]</ref> | |
| − | <ref name=" | + | <ref name="Buono-2007">MJ. Buono, KD. Ball, FW. Kolkhorst, Sodium ion concentration vs. sweat rate relationship in humans., J Appl Physiol (1985), volume 103, issue 3, pages 990-4, Sep 2007, doi [http://dx.doi.org/10.1152/japplphysiol.00015.2007 10.1152/japplphysiol.00015.2007], PMID [http://www.ncbi.nlm.nih.gov/pubmed/17600161 17600161]</ref> |
| − | Na+ secretion rate increases proportionally more than the Na+ reabsorption rate with increases in sweat rate | + | <ref name="Baker-2009">LB. Baker, JR. Stofan, AA. Hamilton, CA. Horswill, Comparison of regional patch collection vs. whole body washdown for measuring sweat sodium and potassium loss during exercise., J Appl Physiol (1985), volume 107, issue 3, pages 887-95, Sep 2009, doi [http://dx.doi.org/10.1152/japplphysiol.00197.2009 10.1152/japplphysiol.00197.2009], PMID [http://www.ncbi.nlm.nih.gov/pubmed/19541738 19541738]</ref> |
| − | http:// | + | <ref name="Fitzpatrick">Fitzpatrick's Dermatology in General Medicine, Chapter 81. Biology of Eccrine, Apocrine, and Apoeccrine Sweat Glands</ref> |
| − | </ref> | + | <ref name="Sigal-1968">CB. Sigal, RL. Dobson, The effect of salt intake on sweat gland function., J Invest Dermatol, volume 50, issue 6, pages 451-5, Jun 1968, PMID [http://www.ncbi.nlm.nih.gov/pubmed/5652824 5652824]</ref> |
| − | + | <ref name="Goulet2012">E. D. B. Goulet, Effect of exercise-induced dehydration on endurance performance: evaluating the impact of exercise protocols on outcomes using a meta-analytic procedure, British Journal of Sports Medicine, volume 47, issue 11, 2012, pages 679–686, ISSN [http://www.worldcat.org/issn/0306-3674 0306-3674], doi [http://dx.doi.org/10.1136/bjsports-2012-090958 10.1136/bjsports-2012-090958]</ref> | |
| − | <ref name=" | + | <ref name="Goulet2011">E. D. B. Goulet, Effect of exercise-induced dehydration on time-trial exercise performance: a meta-analysis, British Journal of Sports Medicine, volume 45, issue 14, 2011, pages 1149–1156, ISSN [http://www.worldcat.org/issn/0306-3674 0306-3674], doi [http://dx.doi.org/10.1136/bjsm.2010.077966 10.1136/bjsm.2010.077966]</ref> |
| − | + | <ref name="Convertino-1996">VA. Convertino, LE. Armstrong, EF. Coyle, GW. Mack, MN. Sawka, LC. Senay, WM. Sherman, American College of Sports Medicine position stand. Exercise and fluid replacement., Med Sci Sports Exerc, volume 28, issue 1, pages i-vii, Jan 1996, PMID [http://www.ncbi.nlm.nih.gov/pubmed/9303999 9303999]</ref> | |
| − | <ref name=" | + | <ref name="WallWatson2013">B. A. Wall, G. Watson, J. J. Peiffer, C. R. Abbiss, R. Siegel, P. B. Laursen, Current hydration guidelines are erroneous: dehydration does not impair exercise performance in the heat, British Journal of Sports Medicine, 2013, ISSN [http://www.worldcat.org/issn/0306-3674 0306-3674], doi [http://dx.doi.org/10.1136/bjsports-2013-092417 10.1136/bjsports-2013-092417]</ref> |
| − | http://www.ncbi.nlm.nih.gov/pubmed/20984571</ref> | + | <ref name="beverageWWW">How to Calculate Your Sweat Rate - Beverage Institute for Health and Wellness, http://www.beverageinstitute.org/article/how-to-calculate-your-sweat-rate/, Accessed on 25 June 2015</ref> |
| − | + | <ref name="Shirreffs-1997">SM. Shirreffs, RJ. Maughan, Restoration of fluid balance after exercise-induced dehydration: effects of alcohol consumption., J Appl Physiol (1985), volume 83, issue 4, pages 1152-8, Oct 1997, PMID [http://www.ncbi.nlm.nih.gov/pubmed/9338423 9338423]</ref> | |
| − | <ref name=" | + | <ref name="ROBERTS-1963">KE. ROBERTS, mechanism of dehydration following alcohol ingestion., Arch Intern Med, volume 112, pages 154-7, Aug 1963, PMID [http://www.ncbi.nlm.nih.gov/pubmed/14044808 14044808]</ref> |
| − | + | <ref name="RubiniKleeman1955">Milton E. Rubini, Charles R. Kleeman, Ezra Lamdin, Studies on alcohol diuresis. I. The effect of ethyl alcohol ingestion on water, electrolyte and acid-base metabolism 12, Journal of Clinical Investigation, volume 34, issue 3, 1955, pages 439–447, ISSN [http://www.worldcat.org/issn/0021-9738 0021-9738], doi [http://dx.doi.org/10.1172/JCI103092 10.1172/JCI103092]</ref> | |
| − | <ref name=" | + | <ref name="Eggleton-1942">MG. Eggleton, The diuretic action of alcohol in man., J Physiol, volume 101, issue 2, pages 172-91, Aug 1942, PMID [http://www.ncbi.nlm.nih.gov/pubmed/16991552 16991552]</ref> |
| − | + | <ref name="Shirreffs-1996">SM. Shirreffs, AJ. Taylor, JB. Leiper, RJ. Maughan, Post-exercise rehydration in man: effects of volume consumed and drink sodium content., Med Sci Sports Exerc, volume 28, issue 10, pages 1260-71, Oct 1996, PMID [http://www.ncbi.nlm.nih.gov/pubmed/8897383 8897383]</ref> | |
| + | <ref name="Maughan-1995">RJ. Maughan, JB. Leiper, Sodium intake and post-exercise rehydration in man., Eur J Appl Physiol Occup Physiol, volume 71, issue 4, pages 311-9, 1995, PMID [http://www.ncbi.nlm.nih.gov/pubmed/8549573 8549573]</ref> | ||
| + | <ref name="Nose-1988">H. Nose, GW. Mack, XR. Shi, ER. Nadel, Role of osmolality and plasma volume during rehydration in humans., J Appl Physiol (1985), volume 65, issue 1, pages 325-31, Jul 1988, PMID [http://www.ncbi.nlm.nih.gov/pubmed/3403476 3403476]</ref> | ||
| + | <ref name="OlssonSaltin1970">Karl-Erik Olsson, Bengt Saltin, Variation in Total Body Water with Muscle Glycogen Changes in Man, Acta Physiologica Scandinavica, volume 80, issue 1, 1970, pages 11–18, ISSN [http://www.worldcat.org/issn/00016772 00016772], doi [http://dx.doi.org/10.1111/j.1748-1716.1970.tb04764.x 10.1111/j.1748-1716.1970.tb04764.x]</ref> | ||
</references> | </references> | ||
Latest revision as of 09:58, 30 September 2024
The science of hydration for exercise is complex and controversial. While dehydration can cause problems, the real risk is Hyponatremia where the level of sodium in the blood is too low. The science shows that sodium losses increase exponentially with sweat rate, so exercise in the heat can result in extreme levels of sodium loss. For a simpler look at hydration, see Practical Hydration.
Contents
1 Introduction
The advice given to runners on hydration has changed over time and looks set to continue to change. There are competing forces at work - sports drink manufacturers, event organizers (often sponsored by the manufacturers) and scientists (some also sponsored by the manufacturers). One thing is clear about hydration - it is important. Incorrect hydration can lead to impaired performance, and in extreme cases, death. A condition related to dehydration is Hyponatremia, which is where the sodium (salt) level in the blood becomes too dilute. This is a dangerous condition that has killed a number of runners. This entry is a follow on to Practical Hydration which should be read first.
2 Effects of dehydration
While it's commonly believed that even small levels of dehydration impacts performance, the research indicates that real world performance is not impacted by dehydration up to 4% of body weight[1][2]. Greater levels of dehydration will impact performance[3]. While authorities have recommended aggressive drinking[4], these guidelines are now recognized as erroneous[5], though they are still promoted by the beverage industry[6].
3 Glycogen Depletion, Dehydration and Body Weight
A common method of calculating dehydration is simply from body weight. While it's true that an athlete's weight loss during exercise will be predominantly from water, this is not the same as dehydration, nor does it necessarily imply this weight needs to be restored quickly though drinking. This is because a carbohydrate (Glycogen) is stored with water, in the ratio of about 1g Glycogen to 3-4g water[7][8]. This means that 2000 calories of Glycogen depletion that are likely to occur in marathon distance runs would result in about 4lb/2Kg Weight Loss with no reduction in hydration (2000Kcal/4=500g Glycogen + 1500g to 2000g water = 2000g to 2500g). To restore the weight lost in endurance exercise the Glycogen reserves must also be restored, something that may take days. Even moving from a high carbohydrate to high fat diet can see 6lb Weight Loss from Glycogen depletion.
4 Sodium Intake and Rehydration
Several studies have shown that drinks containing sodium provide better rehydration by reducing urine output.
- Consuming drinks with 61 mmol/l (~1/2 teaspoon salt per quart/liter) reduced urine output and improved hydration when compared with 23 mmol/l (~1/4 teaspoon salt per quart/liter)[9]
- When comparing drinks with 2, 26, 52 and 100 mmol/l, urine output was inversely proportional to sodium concentration, and the 2 & 26 mmol/l cases resulting in dehydrated subjects after 5.5 hours, where the others were rehydrated[10]. (These cases are roughly equal to water, 1/4, 1/2, 3/4 teaspoon of salt per quart/liter.)
- Drinks containing 0.45g salt/100ml reduced urine output and improved hydration[11]. (0.45g/100ml is about 1/10 teaspoon of salt per quart/liter.)
5 Sodium loss through sweat
The amount of salt that is lost through sweating varies a lot. It varies from individual to individual, and for an individual it will vary depending on fitness and heat acclimation. This means that you may have to experiment with your salt intake, both during and after exercise.
5.1 Anatomy of Sweating
Humans have 2-4 million sweat glands over nearly their whole body surface, and though each is tiny, together they weigh as much as a kidney (~100g)[12]. Sweat glands are most numerous on the soles of the feet. Sweat is produced in two steps[12]:
- A coil generates an ultra-filtrated fluid. This fluid has the same sodium concentration (isotonic) as the blood.
- The fluid travels from the coil up the sweat duct which reabsorbs sodium and chloride (but not potassium). This reabsorption is via active transportation (i.e. it requires energy from ATP). The Glycogen stored in the sweat glands will only support the reabsorption for less than 10 min., so the energy is predominantly supplied by the blood. Glucose is the preferred energy source, though Lactate and pyruvate can also be used. Fatty acids, ketones, and amino acids are very poorly used. The reabsorption process also acidifies the final sweat.
The rate of sweat production depends on the local skin temperature and core body temperature. A rise in the localized skin temperature will produce an increased sweat rate within 60 seconds[12].
5.2 Sodium Loss Table
The table below is based on the research showing that sweat sodium concentration increases with sweat rate. The table below is for a runner who is 174cm/70inches high and weighs 60Kg/132lbs, but you can create a customized chart at Sodium Loss. To check your sweat rate, simply weigh yourself before and after a run. Dropping 1 Kg or 2.2 pounds equates to 1 liter of sweating. (Obviously you need to adjust for any fluid intake and avoid urination.)
| Heat Acclimated | Heat Non-Acclimated | ||||||
|---|---|---|---|---|---|---|---|
| Sweat Rate | Sweat Rate | Sweat Concentration | Sodium Loss | Sodium Loss | Sweat Concentration | Sodium Loss | Sodium Loss |
| (l/hr) | (mg/cm2/min) | (mmol/l) | (mg/hr) | (tsp/hr) | (mmol/l) | (mg/hr) | (tsp/hr) |
| 0.5 | 0.49 | 22 | 249 | 0.1 | 31 | 355 | 0.2 |
| 1 | 0.98 | 32 | 732 | 0.3 | 46 | 1044 | 0.4 |
| 1.5 | 1.47 | 43 | 1450 | 0.6 | 61 | 2066 | 0.9 |
| 2 | 1.96 | 53 | 2402 | 1 | 75 | 3423 | 1.5 |
| 2.5 | 2.45 | 63 | 3589 | 1.5 | 90 | 5113 | 2.2 |
This table is based on the research quoted below showing a linear relationship between sweat rate and sweat sodium concentration.
5.3 Sodium Loss and Sweat Rate
The concentration of sodium in sweat depends on the sweat rate. This is believed to be because the sweat is released with a high sodium concentration, then the sodium is reabsorbed before it reaches the surface. The faster the sweating, the less chance for reabsorption.
5.3.1 Converting per-area sweat rates to whole body sweat rates
We can convert from per-area sweat rates to whole body sweat rates by using a Body Surface Area Calculator. For example, a 135 pound, 70 inch high person has a skin surface area of 1.74 m2, which is 17,400 cm2. Therefore 1 mg/cm2/min is 17,400 mg/min, or 17.4 g/min or 1,044 g/hour, or 1 liter/hour.
5.4 Sodium Loss and Fitness
While some sources suggest that increased fitness reduces the sodium concentration in sweat research[15] shows this is not the case. For both trained and untrained individuals sodium concentration depends mainly on sweat rate. In fact, for a given relative intensity (% of V̇O2max) trained individuals will be performing a greater absolute work rate and therefore have a greater sweat rate and sodium concentration.
5.5 Sodium Loss and Heat Acclimation
A study[16] shows that the sodium concentration of sweat is reduced by Heat Acclimation Training. The study used three bouts of 30 min. of exercise in environmental chamber with 10 min. of rest between each bout.
5.6 Sodium Loss and Skin Temperature
A study[17] of sweating great sodium concentration for different temperatures has shown that sodium reabsorption is greater at high temperatures. Unfortunately the units used in this study are not comparable with other studies. The mechanism behind this is unclear, but the implication is that the sodium concentration of sweat in cooler weather may be higher than expected from the above studies.
6 Sodium Retention
The human body has mechanisms to try to maintaining its sodium balance. Greater sodium intake results in the excess being excreted in the sweat and urine. Conversly, restricted sodium intake will result in a reduction of the sodium concentration of the sweat[18]. This reduction in the sodium concentration occurs at all sweating rates, but the relationship between sodium concentration and sweat rate remains a straight line[19]. So at any given sweat rate, a restricted sodium intake will result in less sodium in the sweat. However, even on a restricted sodium intake, the more you sweat, the greater the sodium concentration. Comparing the maximum sodium concentration of sweat between a salt intake of 500mg/day and 20,000mg/day, the low salt intake reduced the sodium concentration by 30-48%[19].
7 Sodium Intake
Below are some sample sources of Sodium, with the concentrations defined.
| Source | Sodium - mmol per liter | Sodium - grams per liter | Sodium - grams per pint | Salt - grams per pint |
|---|---|---|---|---|
| Gatorade | 18 | 0.4 | 0.2 | 0.5 |
| Water + 1/4 Teaspoon salt per quart | 27 | 0.6 | 0.3 | 0.75 |
| Gatorade+ 1/4 Teaspoon salt per quart | 45 | 1.0 | 0.5 | 1.2 |
| S-Caps + 8oz water* | 65 | 1.4 | 0.7 | 1.7 |
| Salt Stick + 8oz Water | 38 | 0.84 | 0.4 | 0.98 |
| Salt Stick + 16oz Water | 19 | 0.42 | 0.2 | 0.49 |
Note: S-Caps does not specify the amount of fluid to take with each capsule, but does mention 'at least one cup', so this ratio is used. The per-pint and per-liter equivalents assume a constant ratio of one capsule per 8oz of water. See also Comparison of Gels.
7.1 Example Sodium Losses
Here are some hypothetical examples
- Adam, a heat acclimatized runner, weighs himself before and after his four hour run and the difference is 8 pounds, which is roughly equivalent to 8 pints/4 liters of sweat. Based on 1 liter/hour of sweating we estimate Adam lost 4 grams of sodium, which is about 2 teaspoons.
- Bob is not heat acclimatized runner, and losses 9 pounds in three hours (9 pints/4.5 liters). From the sweat rate we estimate that Bob has lost 7.5 grams of sodium, which is about 3.3 teaspoons.
8 Sweat Rates While Running
Sweat rates in male runners have been measured in the range from 0.75-2.23 in winter to 0.99-2.55 in the summer (Liters per hour)[20]. At the low end, we can imagine a fit runner finishing a 3-hour marathon in winter and sweating only 2.25 Liters. Assuming they are also heat acclimated, they would only lose 2 grams of sodium, which is 5 grams of salt, less than a teaspoon. On the other end of the scale, a fit, but unacclimatized runner completing a 5 hour marathon in summer would sweat out nearly 13 Liters, 18 grams of sodium, which is 45 grams of salt or more than 7 teaspoons. There is a table showing a range of values at Sodium Loss.
9 Hyponatremia
Hyponatremia is where the sodium (salt) levels in the blood become too dilute. The symptoms of mild hyponatremia tend to be a gain in weight and a general swelling and 'puffiness', most noticeable in the hands. However, mild Hyponatremia may have no clinical symptoms, or just weakness, dizziness, headache, nausea/vomiting, but more severe Hyponatremia is likely to have symptoms of cerebral edema, including altered mental status, seizures, pulmonary edema, coma, and death[21].
10 HypERnatremia - the opposite of HypOnatremia
Generally, Hypernatremia (too much sodium in the blood) seems to be a result of dehydration rather than excessive salt intake[22]. It should be noted that taking Electrolyte Capsules bypasses the body's taste. This sense of taste seems to reflect our body's internal sensors; our desire for salty foods reflects our salt requirements.
11 Salt and High Blood Pressure
There is evidence that increased salt intake can increase blood pressure[23], and the common recommendation is to restrict your salt intake if you have high blood pressure. However, a recent study[24] has shown that reducing your salt intake may increase your risk of a heart attack rather than lower it. For more on the health risks of low salt diets see http://www.drmirkin.com/public/ezine050811.html As an aside, if you have low blood pressure, which I do, increasing your salt intake can really help.
12 Caffeine
The scientific evidence shows that Caffeine is generally not a diuretic[25][26]. Previous studies have shown that if you don't normally take Caffeine and then get a large dose, there is some diuretic effect. However normal intakes of Caffeine by non-users and use by regular users is not a diuretic[27]. (If you urinate more because you drink a 20oz Latte, it is because of the 20oz of fluid, not the Caffeine.)
13 Alcohol
Alcohol is a diuretic due to the suppression of vasopressin secretion[28][29], and the volume of urine produced is proportional to the alcohol consumed[30]. However, the diuretic effect of alcohol may be reduced when consumed when suffering from post-exercise dehydration. A study looked at consuming water or an alcoholic beverage of 1%, 2%, and 4% after 1 hour of moderate exercise[31]. The study found that urine production tended to increase with alcohol content, but the difference was not statistically significant. The alcohol tended to reduce the rate of recovery for both blood and plasma volume, though tendency was only statistically significant at 4%. In summary, alcoholic beverages of up to 2% appear to have little impact on rehydration rate compared with the equivalent volume of water, but 4% beverages tend to delay recovery.
14 Cramps
The evidence for hydration and electrolyte status causing Cramps is somewhat ambiguous, but supplementing your electrolyte intake may help.
15 Blisters and black toe nails
Dehydration reduces body weight, which can reduce the size of your feet. This in turn changes the fit of your Shoes, causing blisters. Hyponatremia can cause swelling, which increases the size of your feet and can cause blisters. Both conditions can also increase the chance of black toe nails.
16 Sodium and Water in the Body
Approximately 60% of the human body weight is water, though this varies primarily with body fat as adipose (fat) tissue contains a lower percentage of water. Total Body Water (TBW) can be divided up into[32]:
- Intracellular fluid (ICF) which is 40% of body weight
- Extracellular fluid (ECF) which is the other 20% of body weight
- Plasma is 25% of ECF/5% body weight
- Interstitial fluid which is 75% of ECF/15% of body weight, typically 11 Liters/22 pints.
The volume of extracellular fluid is typically 15 liters in a 70 kg human, and the 50 grams of sodium it contains is about 90% of the body's total sodium content.
17 Symptoms of Dehydration
These symptoms are for the general public[32], and there is evidence[33] that they may not apply to athletes suffering from dehydration.
| symptom | mild dehydration (3-5% body weight) | Moderate dehydration (6-9% body weight) | Severe dehydration (>10% body weight) |
|---|---|---|---|
| Level of consciousness | Alert | Lethargic | Obtunded |
| Capillary Refill | 2 seconds | 2-4 seconds | >4 seconds |
| Blood Pressure | Normal | Normal supine, lower standing | lower |
| Skin Turgor | Normal | Slow | Tenting |
| Eyes | Normal | Sunken | Very Sunken |
18 References
- ↑ E. D. B. Goulet, Effect of exercise-induced dehydration on endurance performance: evaluating the impact of exercise protocols on outcomes using a meta-analytic procedure, British Journal of Sports Medicine, volume 47, issue 11, 2012, pages 679–686, ISSN 0306-3674, doi 10.1136/bjsports-2012-090958
- ↑ E. D. B. Goulet, Effect of exercise-induced dehydration on time-trial exercise performance: a meta-analysis, British Journal of Sports Medicine, volume 45, issue 14, 2011, pages 1149–1156, ISSN 0306-3674, doi 10.1136/bjsm.2010.077966
- ↑ J. González-Alonso, R. Mora-Rodríguez, PR. Below, EF. Coyle, Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise., J Appl Physiol (1985), volume 79, issue 5, pages 1487-96, Nov 1995, PMID 8594004
- ↑ VA. Convertino, LE. Armstrong, EF. Coyle, GW. Mack, MN. Sawka, LC. Senay, WM. Sherman, American College of Sports Medicine position stand. Exercise and fluid replacement., Med Sci Sports Exerc, volume 28, issue 1, pages i-vii, Jan 1996, PMID 9303999
- ↑ B. A. Wall, G. Watson, J. J. Peiffer, C. R. Abbiss, R. Siegel, P. B. Laursen, Current hydration guidelines are erroneous: dehydration does not impair exercise performance in the heat, British Journal of Sports Medicine, 2013, ISSN 0306-3674, doi 10.1136/bjsports-2013-092417
- ↑ How to Calculate Your Sweat Rate - Beverage Institute for Health and Wellness, http://www.beverageinstitute.org/article/how-to-calculate-your-sweat-rate/, Accessed on 25 June 2015
- ↑ Karl-Erik Olsson, Bengt Saltin, Variation in Total Body Water with Muscle Glycogen Changes in Man, Acta Physiologica Scandinavica, volume 80, issue 1, 1970, pages 11–18, ISSN 00016772, doi 10.1111/j.1748-1716.1970.tb04764.x
- ↑ LH. Nilsson, Liver glycogen content in man in the postabsorptive state., Scand J Clin Lab Invest, volume 32, issue 4, pages 317-23, Dec 1973, PMID 4771101
- ↑ SM. Shirreffs, AJ. Taylor, JB. Leiper, RJ. Maughan, Post-exercise rehydration in man: effects of volume consumed and drink sodium content., Med Sci Sports Exerc, volume 28, issue 10, pages 1260-71, Oct 1996, PMID 8897383
- ↑ RJ. Maughan, JB. Leiper, Sodium intake and post-exercise rehydration in man., Eur J Appl Physiol Occup Physiol, volume 71, issue 4, pages 311-9, 1995, PMID 8549573
- ↑ H. Nose, GW. Mack, XR. Shi, ER. Nadel, Role of osmolality and plasma volume during rehydration in humans., J Appl Physiol (1985), volume 65, issue 1, pages 325-31, Jul 1988, PMID 3403476
- ↑ 12.0 12.1 12.2 Fitzpatrick's Dermatology in General Medicine, Chapter 81. Biology of Eccrine, Apocrine, and Apoeccrine Sweat Glands
- ↑ MJ. Buono, R. Claros, T. Deboer, J. Wong, Na+ secretion rate increases proportionally more than the Na+ reabsorption rate with increases in sweat rate., J Appl Physiol (1985), volume 105, issue 4, pages 1044-8, Oct 2008, doi 10.1152/japplphysiol.90503.2008, PMID 18653750
- ↑ 14.0 14.1 LB. Baker, JR. Stofan, AA. Hamilton, CA. Horswill, Comparison of regional patch collection vs. whole body washdown for measuring sweat sodium and potassium loss during exercise., J Appl Physiol (1985), volume 107, issue 3, pages 887-95, Sep 2009, doi 10.1152/japplphysiol.00197.2009, PMID 19541738
- ↑ N. Hamouti, J. Del Coso, JF. Ortega, R. Mora-Rodriguez, Sweat sodium concentration during exercise in the heat in aerobically trained and untrained humans., Eur J Appl Physiol, volume 111, issue 11, pages 2873-81, Nov 2011, doi 10.1007/s00421-011-1911-6, PMID 21431880
- ↑ MJ. Buono, KD. Ball, FW. Kolkhorst, Sodium ion concentration vs. sweat rate relationship in humans., J Appl Physiol (1985), volume 103, issue 3, pages 990-4, Sep 2007, doi 10.1152/japplphysiol.00015.2007, PMID 17600161
- ↑ AK. Shamsuddin, T. Kuwahara, A. Oue, C. Nomura, S. Koga, Y. Inoue, N. Kondo, Effect of skin temperature on the ion reabsorption capacity of sweat glands during exercise in humans., Eur J Appl Physiol, volume 94, issue 4, pages 442-7, Jul 2005, doi 10.1007/s00421-005-1354-z, PMID 15843956
- ↑ JW. CONN, MW. JOHNSTON, LH. LOUIS, Relationship between salt intake and sweat salt concentration under conditions of hard work in humid heat., Fed Proc, volume 5, issue 1 Pt 2, pages 230, 1946, PMID 20984571
- ↑ 19.0 19.1 19.2 CB. Sigal, RL. Dobson, The effect of salt intake on sweat gland function., J Invest Dermatol, volume 50, issue 6, pages 451-5, Jun 1968, PMID 5652824
- ↑ http://journals.lww.com/acsm-msse/_layouts/oaks.journals/ImageView.aspx?k=acsm-msse:2007:02000:00022&i=TT2
- ↑ M. H. Rosner, J. Kirven, Exercise-Associated Hyponatremia, Clinical Journal of the American Society of Nephrology, volume 2, issue 1, 2006, pages 151–161, ISSN 1555-9041, doi 10.2215/CJN.02730806
- ↑ A. Kratz, AJ. Siegel, JG. Verbalis, MM. Adner, T. Shirey, E. Lee-Lewandrowski, KB. Lewandrowski, Sodium status of collapsed marathon runners., Arch Pathol Lab Med, volume 129, issue 2, pages 227-30, Feb 2005, doi <227:SSOCMR>2.0.CO;2 10.1043/1543-2165(2005)129<227:SSOCMR>2.0.CO;2, PMID 15679427
- ↑ Micronutrient Information Center - Sodium http://lpi.oregonstate.edu/infocenter/minerals/sodium/
- ↑ K. Stolarz-Skrzypek, T. Kuznetsova, L. Thijs, V. Tikhonoff, J. Seidlerová, T. Richart, Y. Jin, A. Olszanecka, S. Malyutina, Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion., JAMA, volume 305, issue 17, pages 1777-85, May 2011, doi 10.1001/jama.2011.574, PMID 21540421
- ↑ TE. Graham, E. Hibbert, P. Sathasivam, Metabolic and exercise endurance effects of coffee and caffeine ingestion., J Appl Physiol (1985), volume 85, issue 3, pages 883-9, Sep 1998, PMID 9729561
- ↑ B. Falk, R. Burstein, J. Rosenblum, Y. Shapiro, E. Zylber-Katz, N. Bashan, Effects of caffeine ingestion on body fluid balance and thermoregulation during exercise., Can J Physiol Pharmacol, volume 68, issue 7, pages 889-92, Jul 1990, PMID 2383801
- ↑ RJ. Maughan, J. Griffin, Caffeine ingestion and fluid balance: a review., J Hum Nutr Diet, volume 16, issue 6, pages 411-20, Dec 2003, PMID 19774754
- ↑ KE. ROBERTS, mechanism of dehydration following alcohol ingestion., Arch Intern Med, volume 112, pages 154-7, Aug 1963, PMID 14044808
- ↑ Milton E. Rubini, Charles R. Kleeman, Ezra Lamdin, Studies on alcohol diuresis. I. The effect of ethyl alcohol ingestion on water, electrolyte and acid-base metabolism 12, Journal of Clinical Investigation, volume 34, issue 3, 1955, pages 439–447, ISSN 0021-9738, doi 10.1172/JCI103092
- ↑ MG. Eggleton, The diuretic action of alcohol in man., J Physiol, volume 101, issue 2, pages 172-91, Aug 1942, PMID 16991552
- ↑ SM. Shirreffs, RJ. Maughan, Restoration of fluid balance after exercise-induced dehydration: effects of alcohol consumption., J Appl Physiol (1985), volume 83, issue 4, pages 1152-8, Oct 1997, PMID 9338423
- ↑ 32.0 32.1 Clinical Studies in Fluid and Electrolyte Balance
- ↑ J. McGarvey, J. Thompson, C. Hanna, T. D. Noakes, J. Stewart, D. Speedy, Sensitivity and specificity of clinical signs for assessment of dehydration in endurance athletes, British Journal of Sports Medicine, volume 44, issue 10, 2008, pages 716–719, ISSN 0306-3674, doi 10.1136/bjsm.2008.053249